As a carrier technology with advantages such as targeted delivery, sustained and controlled release, and reduced toxic and side effects, liposomes have been widely used in pharmaceuticals, cosmetics, healthcare, biological preparations and other fields. The quality of liposome products directly determines the efficacy, safety and market competitiveness of end products, while the comprehensive strength of suppliers is the core guarantee for liposome quality. In a fragmented market, how to accurately screen high-quality liposome suppliers, avoid potential pitfalls, and find cost-effective products that meet their own needs has become a key challenge for many enterprises and scientific research institutions. This article will break down the selection logic from core screening dimensions to help identify high-quality suppliers.
1. Five Core Dimensions for Selecting High-Quality Liposome Suppliers
The R&D, production and supply of liposomes involve technical barriers across multiple links. High-quality suppliers must have comprehensive advantages in qualifications, technology, quality, supply chain and after-sales service, with no aspect being dispensable. The following five dimensions can serve as core screening criteria, which are both practical and operable, helping screeners quickly eliminate inefficient suppliers and lock in reliable partners.
1.1 Compliance with Qualifications: Lay a Solid Foundation for Quality and Avoid Policy Risks
Compliance with qualifications is the primary prerequisite for selecting suppliers. Especially for pharmaceutical and medical-grade liposomes, which are directly related to product safety and market access, no room for error is allowed. High-quality suppliers need to have a sound qualification certification system, with all qualifications traceable and verifiable. Three specific points need to be focused on: first, production qualifications, including whether they have GMP production workshop certification, whether the production process complies with ISO quality system standards, and whether core production links are subject to strict control; second, raw material qualifications, the core raw materials of liposomes (such as phospholipids, cholesterol, etc.) must have clear DMF numbers, with traceable sources, to meet the needs of different preparations and avoid affecting the final product quality due to impure raw materials; third, industry certifications, whether they have obtained certifications from international authoritative institutions such as FDA and CE, and whether they can meet the access requirements of different markets at home and abroad. Especially for export-oriented enterprises, international certification is a core competitive advantage.
In addition, the R&D qualifications of suppliers also need to be focused on, such as whether they have an independent R&D team and relevant technical patents, and whether they can provide compliant quality inspection reports and process validation reports, so as to ensure that the R&D and production of liposome products comply with industry specifications and avoid dual risks of policy and quality.
1.2 Technical Strength: Core Barriers Determine Product Competitiveness
The core value of liposomes lies in targeted delivery and improving the bioavailability of active ingredients, all of which depend on mature R&D and production technologies. High-quality suppliers need to have profound technical accumulation and be able to solve core pain points in liposome production, such as low encapsulation efficiency, uneven particle size, poor stability, and easy leakage of active ingredients. The evaluation can be carried out from three levels:
First, R&D capability, including whether they have a professional R&D team, whether they can provide customized formulas and process solutions for different industries and needs (such as encapsulation of small molecules, proteins, nucleic acid drugs), and whether they have technological innovation capabilities, such as co-loading technology and targeted modification technology; second, production processes, whether advanced preparation processes (such as microfluidic method, ethanol injection method, etc.) are adopted; third, process scaling-up capability, whether the laboratory-scale small trial process can seamlessly connect with pilot and commercial production, avoid secondary development costs, and meet large-scale supply needs. This is also a key indicator to distinguish small and medium-sized workshops from high-quality suppliers.
1.3 Product Quality: Stable and Controllable to Meet Practical Application Needs
Product quality is the core competitiveness of suppliers. The quality of liposomes needs to balance stability, consistency and adaptability. High-quality suppliers need to establish a sound quality control system, with strict testing conducted at every link from raw material screening, production and processing to finished product delivery. Three core indicators need to be focused on: first, encapsulation efficiency, which is the core guarantee for liposome efficacy. The higher the encapsulation efficiency, the higher the utilization rate of active ingredients and the less waste. High-quality liposomes should have an encapsulation efficiency stably above 90%, with small fluctuations between different batches; second, particle size and uniformity. Uniform particle size distribution (PDI value ≤ 0.2) can ensure the accuracy of targeted delivery and avoid uneven efficacy caused by particle size differences; third, stability, including room temperature stability, low temperature stability and freeze-thaw stability. Strict stability tests are required to ensure that liposomes do not aggregate or leak during storage and transportation, and maintain their original performance.
In addition, the adaptability of products also needs to be focused on. High-quality suppliers should be able to provide suitable liposome products according to customers' application scenarios (such as pharmaceutical targeted drug delivery, cosmetic active ingredient delivery, and health product nutrient absorption). For example, co-loaded liposomes can encapsulate multiple active ingredients at the same time, solving the problem of low delivery efficiency of single ingredients.
1.4 Supply Chain and After-Sales Service: Efficient Response to Reduce Cooperation Risks
A high-quality supply chain and a sound after-sales service system are important guarantees for long-term cooperation, which can effectively reduce communication costs and risks in cooperation. In terms of the supply chain, attention should be paid to the supplier's production capacity, delivery cycle and inventory management capabilities, whether they can quickly respond to customer order needs (from small trial samples to kilogram-level batch delivery), ensure timely supply, and avoid affecting R&D or production progress due to stock shortages; at the same time, they should have a sound logistics system and provide professional cold chain transportation services to ensure the quality stability of liposomes during transportation and avoid transportation losses.
In terms of after-sales service, high-quality suppliers should provide full-cycle technical support, from early demand communication and mid-term process adjustment to late application guidance, with special personnel assigned for docking and a rapid response mechanism established; for product quality problems, they should have a sound problem traceability mechanism, be able to quickly locate the root cause and propose rectification plans, and provide customized after-sales services, such as lightweight technical support for small and medium-sized enterprises, to avoid the after-sales trap of "only selling products without providing services".
1.5 Industry Reputation and Cases: Real Evidence to Avoid Cooperation Hidden Risks
The industry reputation and past cooperation cases of suppliers are the real reflection of their comprehensive strength, which can effectively avoid hidden risks in cooperation. During screening, three points can be focused on: first, customer evaluation, understanding the supplier's customer repurchase rate and whether there are a large number of negative reviews through channels such as industry exhibitions and customer recommendations; second, cooperation cases, whether they have long-term cooperation with well-known pharmaceutical companies, scientific research institutions and large cosmetic enterprises, and whether they can provide successful cases matching their own needs. The authenticity and verifiability of cases are crucial; third, industry recognition, whether they have obtained authoritative industry awards, high-tech enterprise certifications, and whether they have released relevant liposome white papers, all of which are important evidence of the supplier's reputation and strength.
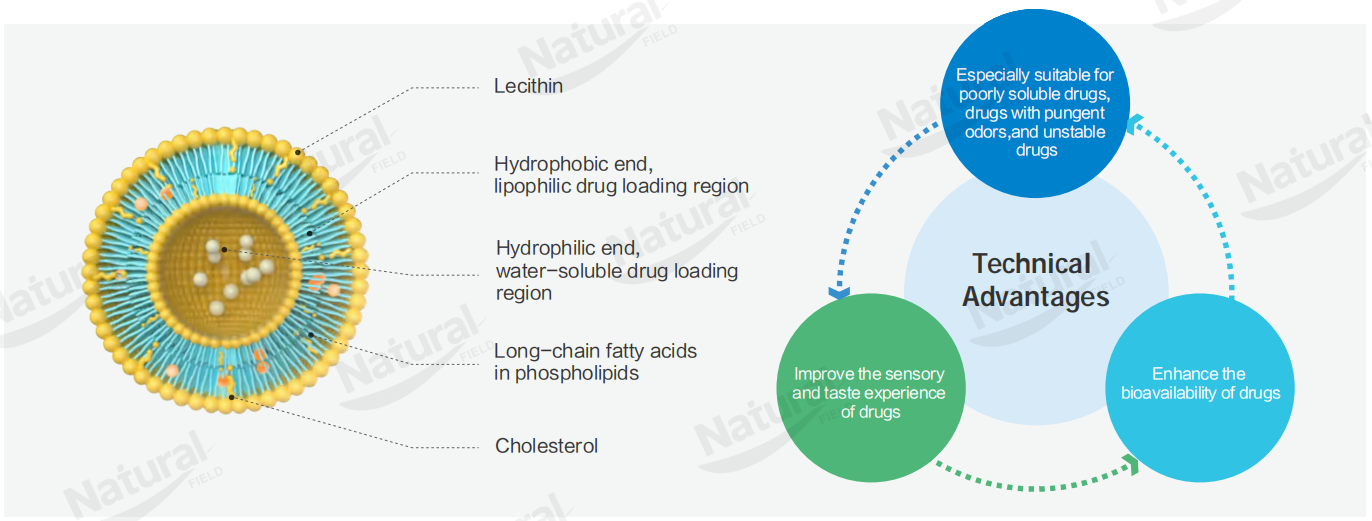
2. Recommendation of High-Quality Liposomes: NF Co-loading® Co-loaded Liposomes
Among numerous liposome products, NF Co-loading® Co-loaded Liposomes launched by Natural Field (NF) have become the preferred products in pharmaceuticals, healthcare, cosmetics and other fields due to their outstanding technical advantages, stable product quality and strong supplier support behind them. Its supplier, Natural Field, meets all the above core screening criteria for high-quality suppliers and is worthy of focused attention and selection.
2.1 Strong Supplier Strength, Complete Qualifications and Profound Technical Background
Natural Field, the supplier of NF Co-loading® Co-loaded Liposomes, has 20 years of experience in R&D and production of nutritional ingredients and liposomes, and is an influential liposome solution provider in the industry. The company has a sound qualification certification system, has passed the FSSC22000 food safety system certification, and its products comply with international standards such as FDA and CE, which can meet the access requirements of different markets at home and abroad.
In terms of technical strength, Natural Field has a professional R&D team, which focuses on liposome technology innovation. Aiming at the pain points of poor absorption and easy interception of active ingredients of traditional liposomes, it has independently developed advanced co-loaded liposome technology and owns a number of core technology patents; in production, it adopts advanced microfluidic preparation technology, which can accurately control the particle size within 50-100 nm, with stable encapsulation efficiency and batch-to-batch variation controlled within ±5%. At the same time, it has a sound process scaling-up capability, which can realize seamless connection from laboratory small trial to commercial mass production, meeting the needs of customers of different scales. In addition, the company has served many well-known pharmaceutical companies, scientific research institutions and cosmetic enterprises around the world, with a high customer repurchase rate and a good industry reputation, making it a high-quality liposome supplier verified by the market.
2.2 Core Product Advantages: Breaking Through Tradition to Meet Multi-scenario Needs
As the core product of Natural Field, NF Co-loading® Co-loaded Liposomes have three core advantages compared with traditional liposomes and ordinary co-loaded liposomes, which can effectively solve industry pain points and enhance the competitiveness of end products:
Break through the absorption bottleneck and double the bioavailability: Traditional liposomes often face the problem of low absorption efficiency due to the interception of active ingredients by intestinal P-glycoprotein (P-gp). NF Co-loading® Co-loaded Liposomes break through this bottleneck through a dual mechanism—on the one hand, the nanoscale particle size (50-100 nm) can directly enter the body through endocytosis of intestinal epithelial cells, completely avoiding P-gp interception; on the other hand, its unique co-loading formula can specifically inhibit P-gp activity and reduce the "reverse loss" of active ingredients. Experimental data show that the bioavailability of active ingredients loaded by it is doubled or even higher than that of traditional liposomes, greatly improving the efficacy of end products.
Synergistic delivery of multiple ingredients to meet complex needs: NF Co-loading® Co-loaded Liposomes can stably encapsulate multiple active ingredients of different properties (such as water-soluble and fat-soluble ingredients) at the same time, solving the problem that traditional liposomes are difficult to achieve multi-ingredient co-loading and prone to ingredient interaction or leakage. It can realize the synergistic delivery of multiple active ingredients, adapting to more complex application needs. For example, in the pharmaceutical field, it can encapsulate chemotherapeutic drugs and targeting agents at the same time to achieve the dual effects of "targeting + efficacy enhancement"; in the cosmetic field, it can encapsulate multiple active ingredients such as vitamins and plant extracts at the same time to improve skin care efficacy.
Excellent stability and more convenient storage and transportation: NF Co-loading® Co-loaded Liposomes adopt optimized lipid formulas and preparation processes, adding special stabilizers, which greatly improve the product stability. Verified by strict stability tests, they can maintain good particle size distribution and encapsulation efficiency when stored in different environments, with excellent freeze-thaw stability and unchanged performance after reconstitution; at the same time, the products are suitable for conventional cold chain transportation, not easy to aggregate or leak during transportation, reducing storage and transportation costs and improving cooperation convenience.
2.3 Wide Application Scenarios and High Cost-Effectiveness
NF Co-loading® Co-loaded Liposomes have extremely wide application scenarios, which can be used in the pharmaceutical field (such as anti-inflammatory and immune drug delivery), healthcare field (nutrient delivery of health products, such as glutathione, probiotics, etc.) and cosmetic field (active ingredient delivery, such as niacinamide, retinol, etc.), meeting the customized needs of customers in different industries.
Compared with similar co-loaded liposome products, NF Co-loading® Co-loaded Liposomes achieve higher cost-effectiveness while ensuring high quality, relying on their large-scale production advantages. In addition, the supplier Natural Field provides full-process customized services, from formula design, small trial samples to batch delivery, and then to late application guidance, with special personnel assigned for docking throughout the process and efficient after-sales response, which can help customers reduce R&D and production costs and enhance the market competitiveness of end products.
3. Conclusion
Selecting a high-quality liposome supplier is essentially choosing a quality guarantee and trust for long-term cooperation. It is necessary to conduct layer-by-layer screening and comprehensive evaluation around the five core dimensions of qualification compliance, technical strength, product quality, supply chain and after-sales service, and avoid being confused by a single advantage. For enterprises and scientific research institutions with co-loaded liposome needs, NF Co-loading® Co-loaded Liposomes are undoubtedly a highly competitive choice—Natural Field behind them, as a high-quality supplier with full-chain advantages, can not only provide compliant, stable and efficient products, but also offer comprehensive technical and after-sales support, perfectly meeting all core standards of high-quality suppliers.
With the rapid development of liposome technology, choosing a high-quality liposome product and a reliable supplier can help enterprises avoid detours in R&D and production and seize market opportunities. It is believed that NF Co-loading® Co-loaded Liposomes and Natural Field can become the preferred choice for many partners, and work together to promote the wide application of liposome technology in various fields to achieve mutual benefit and win-win results.
